Microarray Testing Reliability: Critical Considerations for Clinical Practice
Understanding the technical limitations of antibody microarray platforms and their implications for patient care.
The appeal of microarray-based antibody testing—offering hundreds of results at a fraction of traditional testing costs—is attractive to practitioners looking to find cost-effective solutions for their patients. While these platforms promise comprehensive immune profiling from a single sample, the inherent reliability concerns warrant careful consideration when interpreting results and making clinical decisions.
Understanding Antibody Microarray Technology
Antibody microarrays (protein microarrays or immunoassays on chips) are high-throughput platforms designed to detect proteins, cytokines, and antibodies in biological samples. The technology involves spotting hundreds of different antibodies onto a single chip, enabling simultaneous screening for multiple immune responses.
While the multiplexing capability appears advantageous, the underlying technology has significant limitations that can compromise diagnostic accuracy and clinical utility.
Primary Technical Limitations
Cross-Reactivity and Non-Specific Binding
Cross-reactivity represents the most significant challenge in microarray testing. Antibodies bind to unintended proteins with similar structural epitopes, resulting in false positives that mislead clinical interpretation. This is particularly problematic in food sensitivity panels, autoimmune testing, and inflammatory marker assessments where proteins share structural homology.
Antibody Performance Variability
Microarrays utilize antibodies with varying binding affinities, creating inconsistent performance:
Low-affinity antibodies miss clinically relevant concentrations (false negatives)
High-affinity antibodies oversaturate signals, compromising quantitative accuracy
Batch-to-batch variations significantly affect reproducibility
Manufacturing and Sample Issues
The physical manufacturing process introduces systematic variability through uneven antibody spotting, surface chemistry variations, and storage degradation. Additionally, biological samples contain interfering substances (high-abundance proteins, heterophilic antibodies, lipemia) that can skew results, while sample handling factors like freeze-thaw cycles and delayed processing further compromise protein integrity.
Reproducibility and Standardization Challenges
Batch Effects
Inter-batch variability represents a critical limitation:
Manufacturing variations between array batches
Reagent lot-to-lot differences affecting signal detection
Scanner calibration variations between instruments
Environmental conditions during processing
Lack of Standardization
Unlike established immunoassays, microarray platforms lack:
Standardized reference materials for calibration
Harmonized data processing protocols
Validated cut-off values for clinical interpretation
Proficiency testing programs for quality assurance
Clinical Implications and Diagnostic Accuracy
Food Sensitivity Testing Considerations
The application of microarray technology to food sensitivity testing presents particular challenges:
Cross-reactivity between food proteins can generate false positives
Unnecessarily strict elimination diets based on false positives
Recommendations for Clinical Practice
Result Interpretation Guidelines
Consider the clinical context - symptoms should correlate with test findings
Evaluate the number of positive results - extensive positivity may indicate cross-reactivity
Assess result patterns - related proteins showing similar results suggest technical issues
Correlate with established biomarkers - confirm significant findings with validated tests
When to Consider Confirmation Testing
High-impact clinical decisions (e.g., autoimmune disease diagnosis, prescription or extensive intervention)
Unexpected or numerous positive results
Discordance between symptoms and test results
Prior to significant dietary or therapeutic interventions
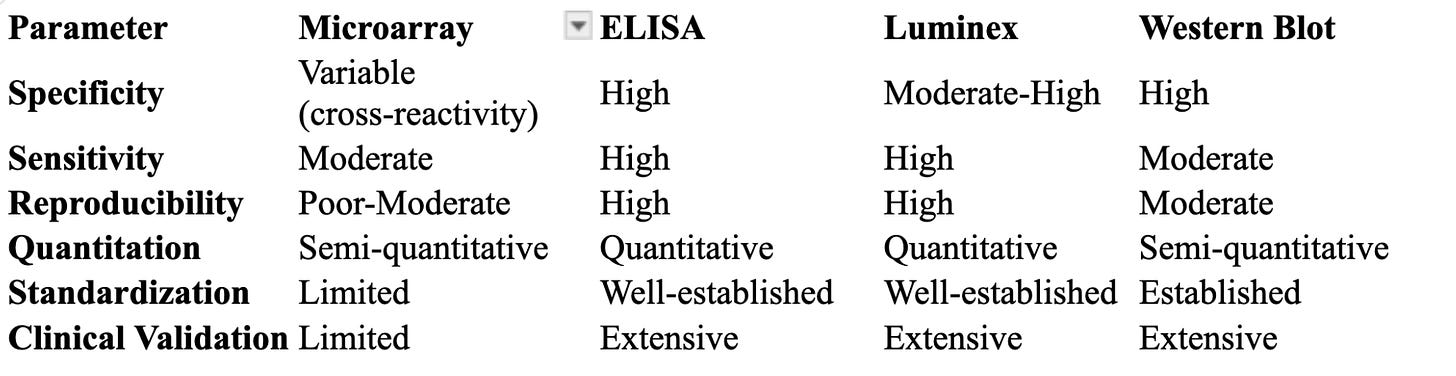
When to Choose Established Methods
ELISA for single-target, high-accuracy quantitation
Luminex multiplex assays for validated multi-target screening
Western blot for confirmatory testing of autoantibodies
Potential Use Cases for Microarray Technology
Research applications for biomarker discovery
Broad screening when subsequently validated by confirmatory testing
Conclusion
While microarray technology offers the appeal of comprehensive immune profiling, its inherent technical limitations pose significant challenges for clinical application. The high risk of false positives due to cross-reactivity, combined with lower reproducibility and less standardization, makes these platforms challenging for reliability.
As practitioners, thoughtful evaluation of microarray results helps us prioritize accuracy and clinical relevance over convenience or cost. When significant clinical decisions are warranted, confirmation through established, validated methods advances diagnostic precision and drives clinical excellence.


Thanks Dr Kristine Burke for your insight and leadership on this topic!